What if I told you that atoms, which we've all known and studied to be particles, actually exhibit properties of both a particle and a wave?
This duality of atoms and other subatomic entities is explained in a branch of physics known as quantum physics. By definition, Quantum physics deals with the behavior of electrons, protons, and other subatomic particles on a microscopic scale.
During the 18th and early 19th centuries, when scientists started studying atoms, they discovered that the laws of classical physics didn't work anymore. Classical physics has well described our macroscopic world, the world that you and I are very familiar with, for example, Newton gave us certain laws that explain how everything works in our daily lives. However, as classical physics did not apply to atoms and the subatomic particles in the microscopic world, scientists needed a new approach. It was then that the development of quantum mechanics was initiated.
It began around the same time as Albert Einstein published his theory of relativity, a different revolution in physics that describes the motion of objects at high speeds. However, unlike relativity, quantum mechanics cannot be traced back to a single scientist. Rather, throughout the late 1800s and 1930s, several scientists contributed to a foundation that progressively acquired recognition and experimental proof. [1]
With the development of quantum physics, many experiments were conducted that gave rise to many theories surrounding the subject, one of such theories is the ‘wave-particle duality theory’
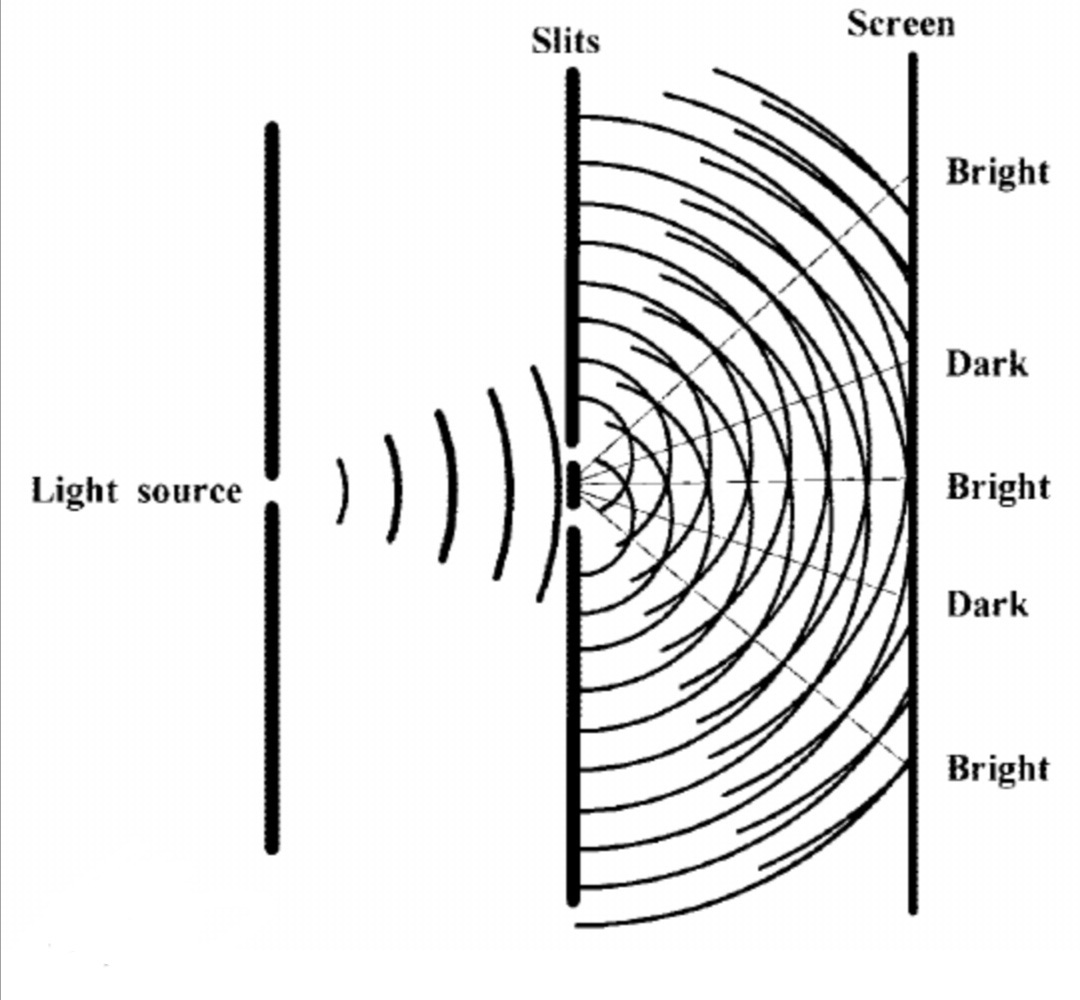
Thomas Young’s double-slit experiment
The original double-slit experiment, performed in 1801 by Thomas Young at the Royal Institution, showed that light acts as a wave. Further experiments, however, showed that light actually behaves as both a wave and as particles – revealing its quantum nature.[2]
[3]
Young's double-slit experiment
In this experiment, a beam of monochromatic light is fired at a barrier with two vertical slits, it is diffracted into two new waves as it emerges from the two slits, the waves spread out and interact with each other. This interaction is known to be either constructive or destructive:
Constructive interaction, meaning the waves intersect both at their peaks or troughs, causing them to amplify and form a bright section of light on the back screen.
Destructive interaction, when the waves intersect while one is at its peak and the other at its trough, this causes them to cancel each other out and form a dark section on the back screen.
These interferences resulted in a series of light and dark segments on the back screen, this pattern became known as the ‘interference pattern’. According to Thomas Young and a few other scientists, this pattern proves that light is a series of waves rather than particles.
Young's view of light persisted until the early 20th century when the theories of quantum mechanics began to take hold. From these theories came the idea that photons, electrons, and other subatomic materials possess a quality called wave-particle duality, which describes them as both waves and particles rather than one or the other. The double-slit experiment has come to play a vital role in demonstrating wave-particle duality and other quantum properties[4]
Scientists continued to conduct similar experiments to advance their understanding of the quantum nature of particles.
The double-slit Experiment with electrons
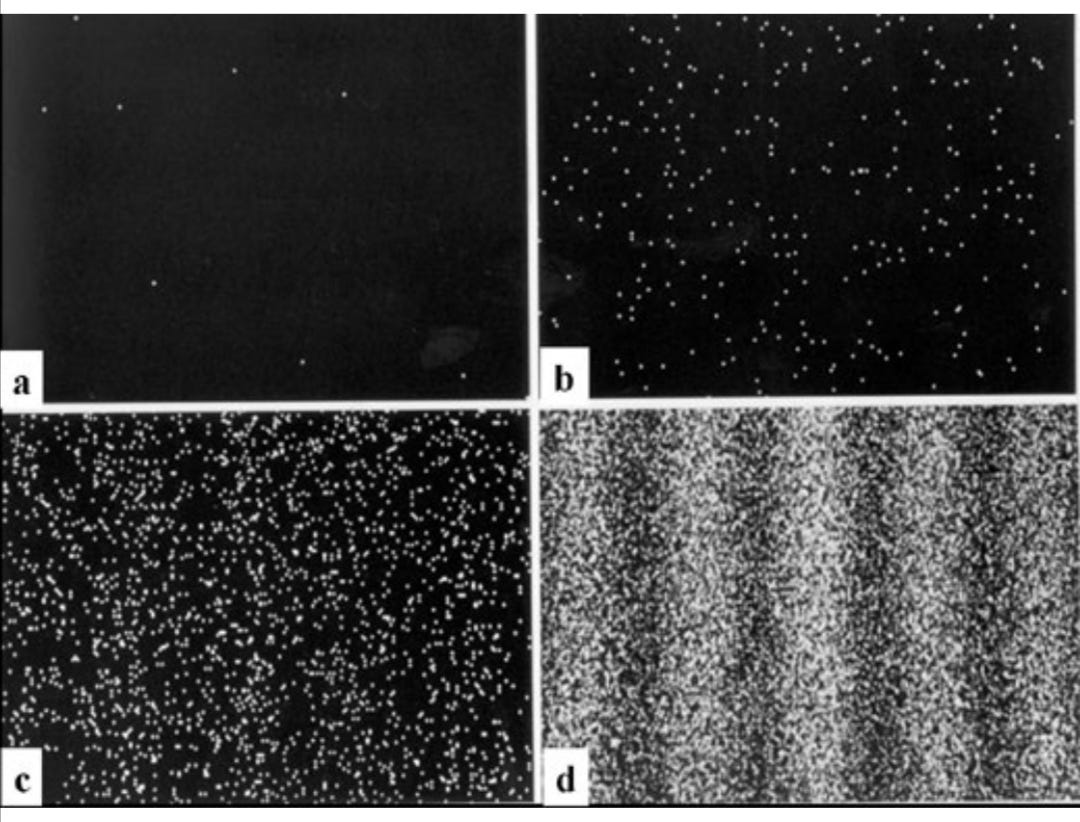
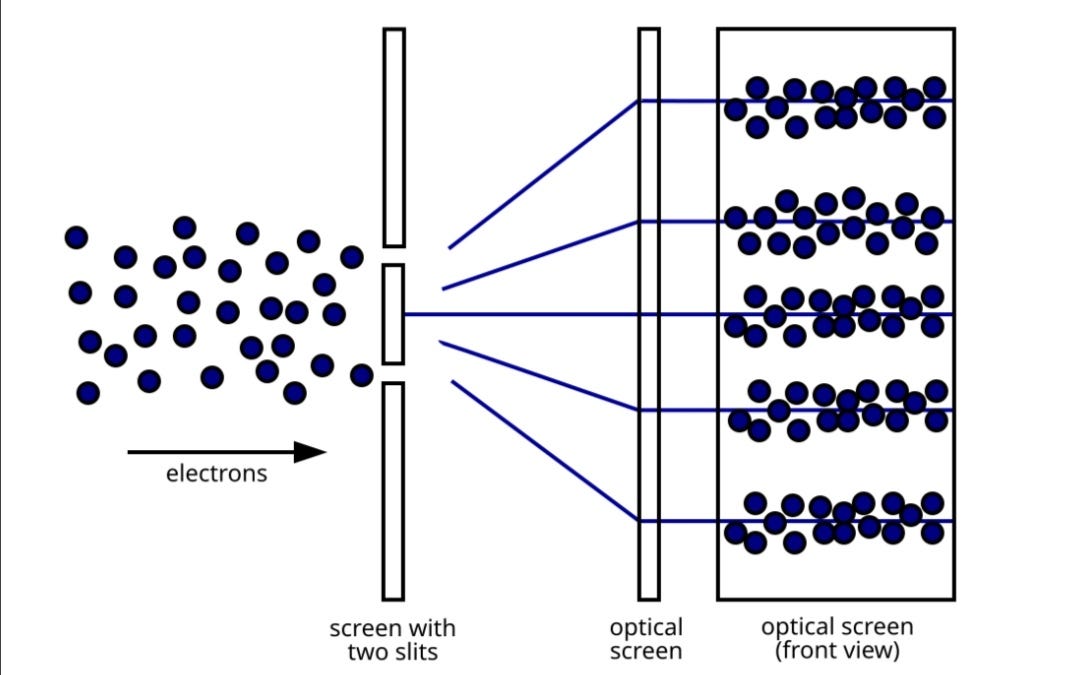
In the more modern version of the double slit experiment using electrons, electrons with the same momentum are shot from an "electron gun" like the ones inside CRT televisions towards a screen with two slits in it. After each electron goes through one of the slits, it is observed hitting a single point on a detecting screen at an apparently random location. As more and more electrons pass through, one at a time, they form an overall pattern of light and dark interference bands [5]
[6]
Appearance of the electrons on the back screen
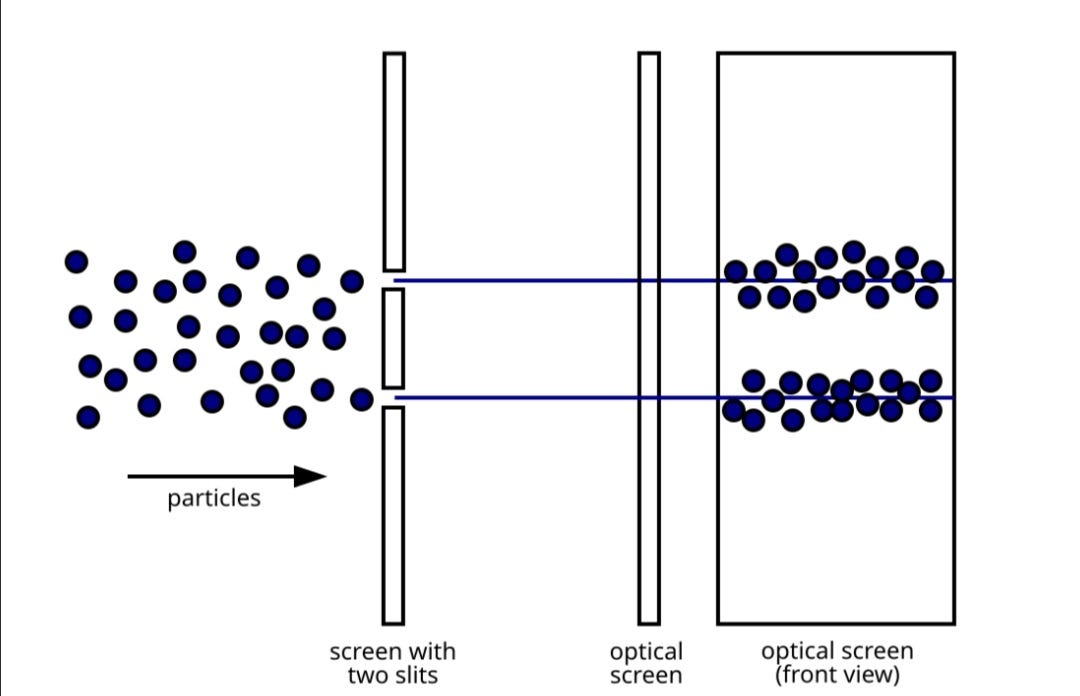
If electrons were strictly particles, we'd expect the electrons being fired in a straight line through the two slits to form a pattern corresponding to the shape of the slit as shown in the image below;
[7]
However, the results looked a bit like this;
[8]
The detecting screen shows numerous circular spots i.e. the electrons, clustered in sections, much like how the light was divided into brighter and darker sections in Young’s experiment. This shows that the electron beam from the electron gun results in the same diffraction and interaction patterns as the beam of light did in Young’s experiment and the circular spots observed at the end of the experiment give evidence of the electron being both a particle and a wave. Similar results were obtained when experiments were conducted using neutrons and the atom as a whole. This proves that atoms which make up the entirety of our universe have dual properties of both waves and particles.
In conclusion, quantum objects show dual properties of both waves and particles, the wave-particle duality theory expresses the inability of classical concepts of the particle or the wave to completely describe the behavior of quantum entities.
Sources
[1] “Overview of history of quantum mechanics” https://unacademy.com/content/csir-ugc/study-material/mathematical-sciences/history-of-quantum-mechanics/
(Accessed on 26.06.24)
[2] “Double-slit experiment that proved the wave nature of light explored in time”
by Thomas Angus [Photographer] and Hayley Dunning
(Accessed on 26.06.24)
[3] “image uploaded by Alasdair N Beal”
(Accessed on 29.06.24)
[4] “What is the double-slit experiment?” https://www.techtarget.com/whatis/definition/double-slit-experiment
(Accessed on 26.06.24)
[5] “Double-slit experiment using electrons”
https://brilliant.org/wiki/double-slit-experiment/
(Accessed on 26.06.24)
[6] “Double-slit experiment; quantum measurement” https://www.hitachi.com/rd/research/materials/quantum/doubleslit/index.html
(Accessed on 29.06.24)